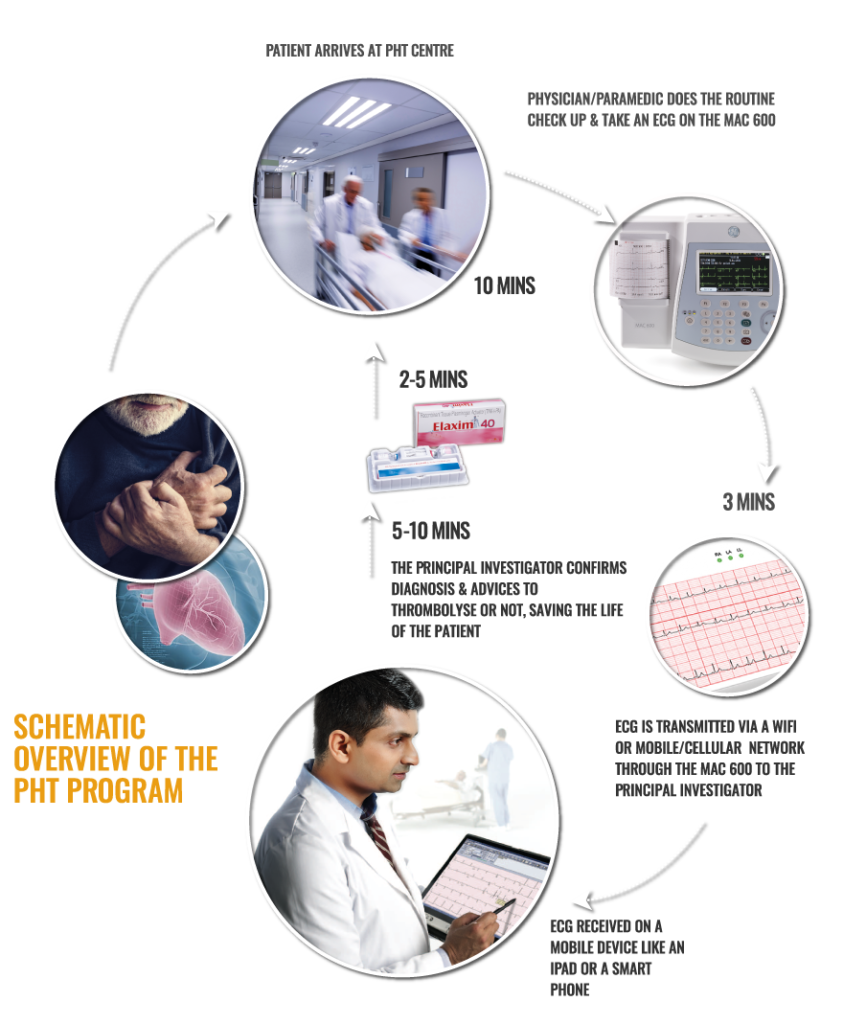
Heart attack Emergency Assuaged by Remote diagnosis and local Treatment
- Home
- Home
- Contact Us
- Careers
- About Us
- Media
- Products
- Publications
- Abhyudaya
- Transforming Healthcare
- Transforming Healthcare
- Global Health Initiative
- Global Health Initiative
- PHT
- Home
- Home
- Contact Us
- Careers
- About Us
- Media
- Products
- Publications
- Abhyudaya
- Transforming Healthcare
- Transforming Healthcare
- Global Health Initiative
- Global Health Initiative
- PHT